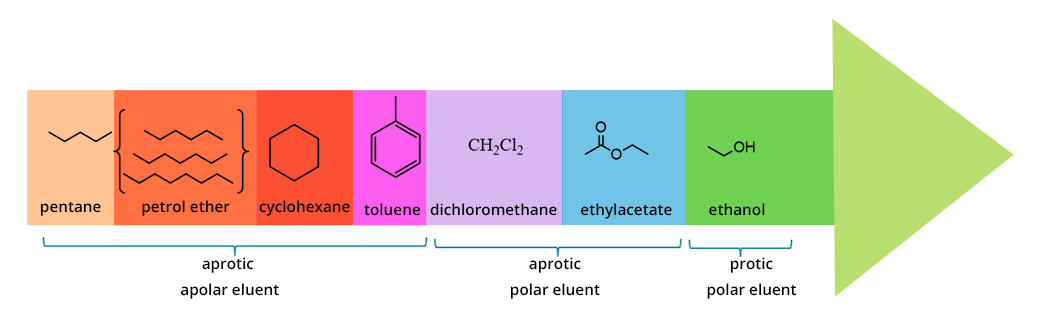
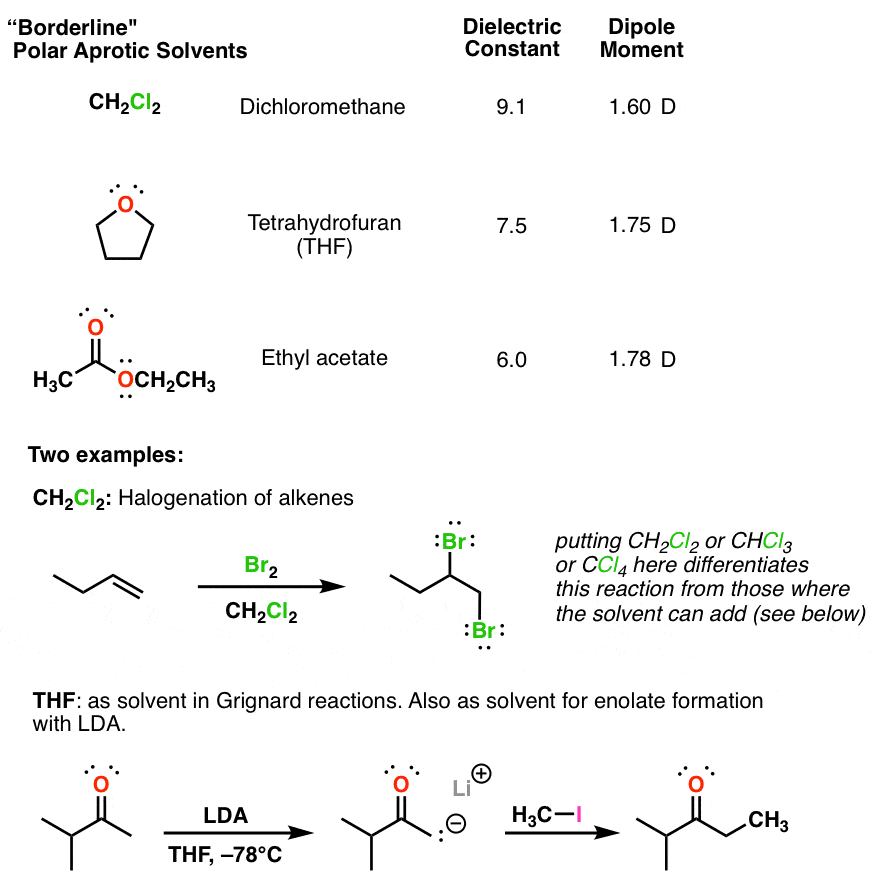
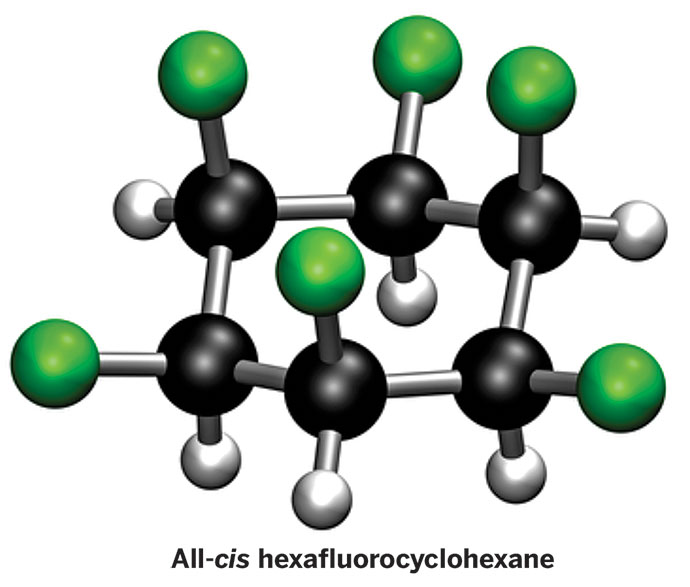
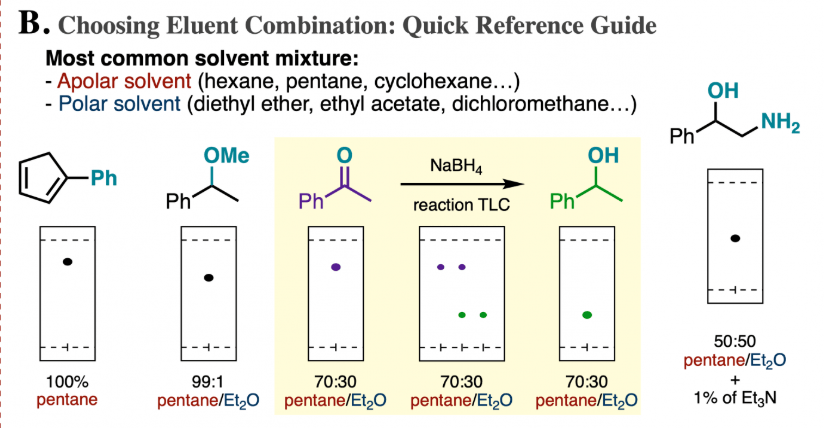
Is cyclohexane an ionic, molecular nonpolar, or molecular polar compound? What intermolecular forces are present? | Homework.Study.com

Intermolecular forces – dipole – dipole forces Lesson Objectives: To describe the interaction of molecules by permanent dipole – dipole To compare dipole. - ppt download

Influence of Solvent Polarity and Hydrogen Bonding on the Electronic Transition of Coumarin 120: A TDDFT Study - Zhao - 2008 - ChemPhysChem - Wiley Online Library