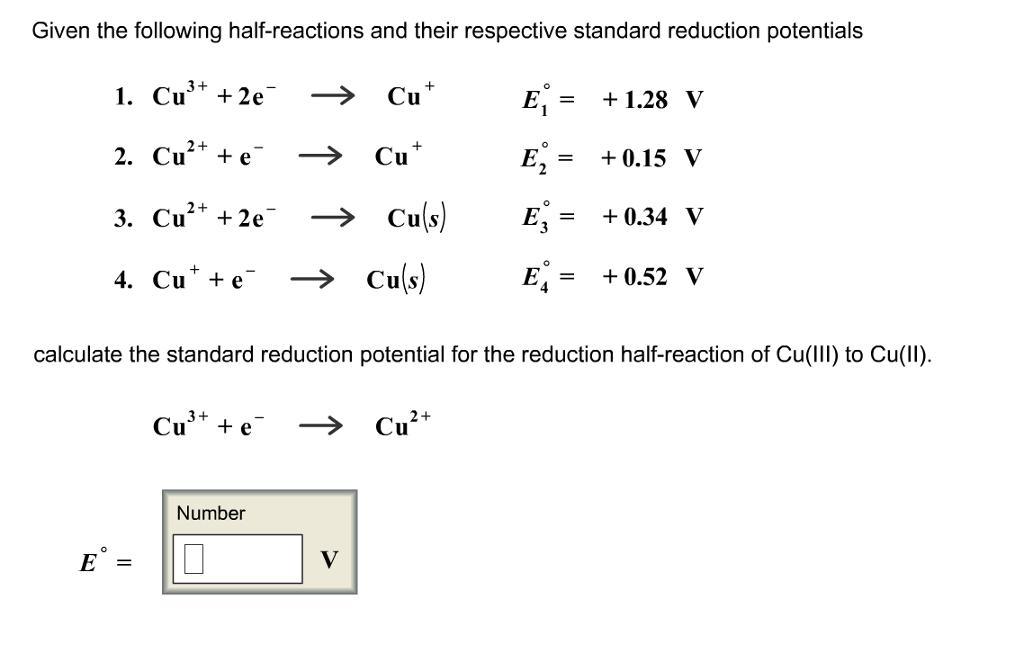
OneClass: Standard reduction potential help! Bicarbonate deprotonates in water with the formation of ...

Calculation of Standard Reduction Potentials of Amino Acid Radicals and the Effects of Water and Incorporation into Peptides. | Semantic Scholar

The standard reduction potential for `Cu^(2+)|Cu` is `+0.34V`. Calculate the reduction potential... - YouTube

The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .
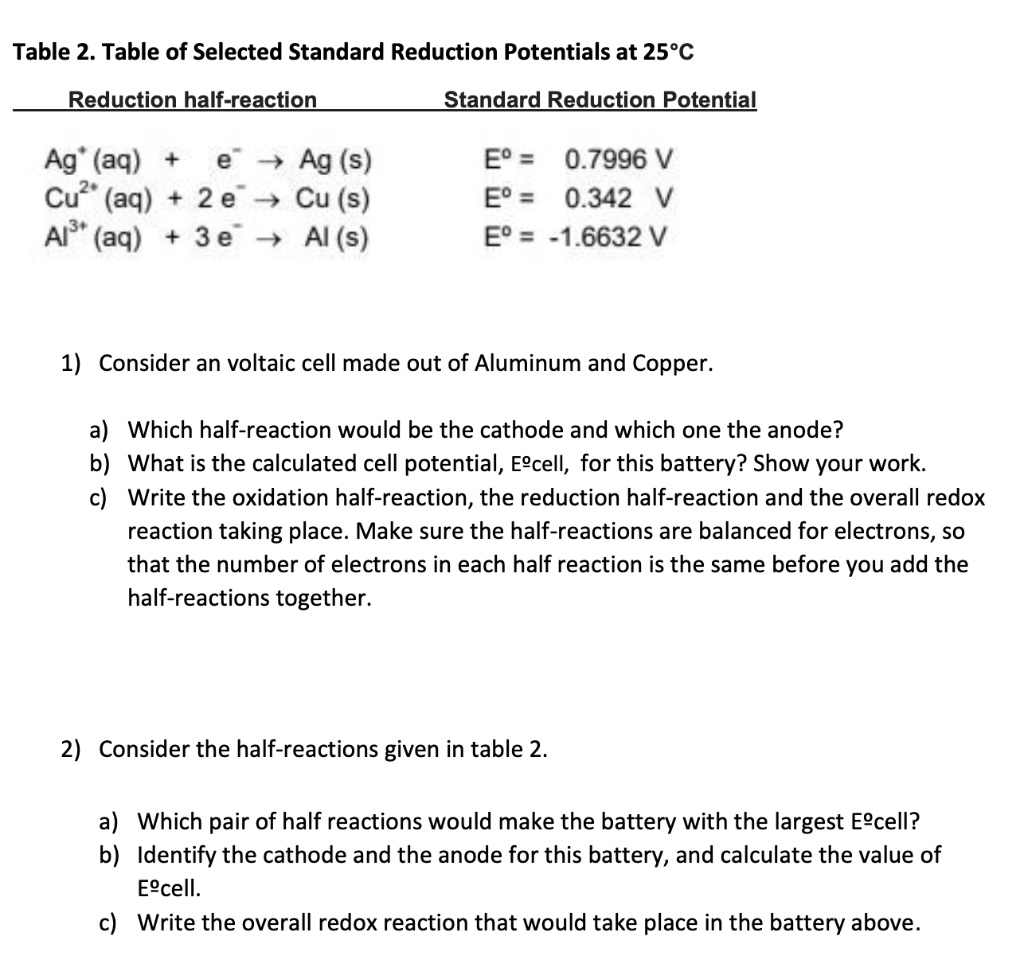
SOLVED: Table 2. Table of Selected Standard Reduction Potentials at 25*€ Reduction half-reaction Standard Reduction Potential Ag' (aq) e 4 Ag (s) Cu?" (aq) 2 e 4 Cu (s) Al"' (aq) 3
2 The standard half reduction potential of Ag+|Ag is 0.79V is 25^° C. Given the experimental value Ksp=1.5 10* 10 for AgCl, calculate the standard half cell reduction potential for the Ag|AgCl
![The standard reduction potential for Cu^2 + /Cu is + 0.34 V. What will be the reduction potential at pH = 14 ? [Given: Ksp of Cu(OH)2 is 1.0 × 10^-19] . The standard reduction potential for Cu^2 + /Cu is + 0.34 V. What will be the reduction potential at pH = 14 ? [Given: Ksp of Cu(OH)2 is 1.0 × 10^-19] .](https://dwes9vv9u0550.cloudfront.net/images/8469704/978f411d-2ca9-41b2-ac30-64ae7a891629.jpg)


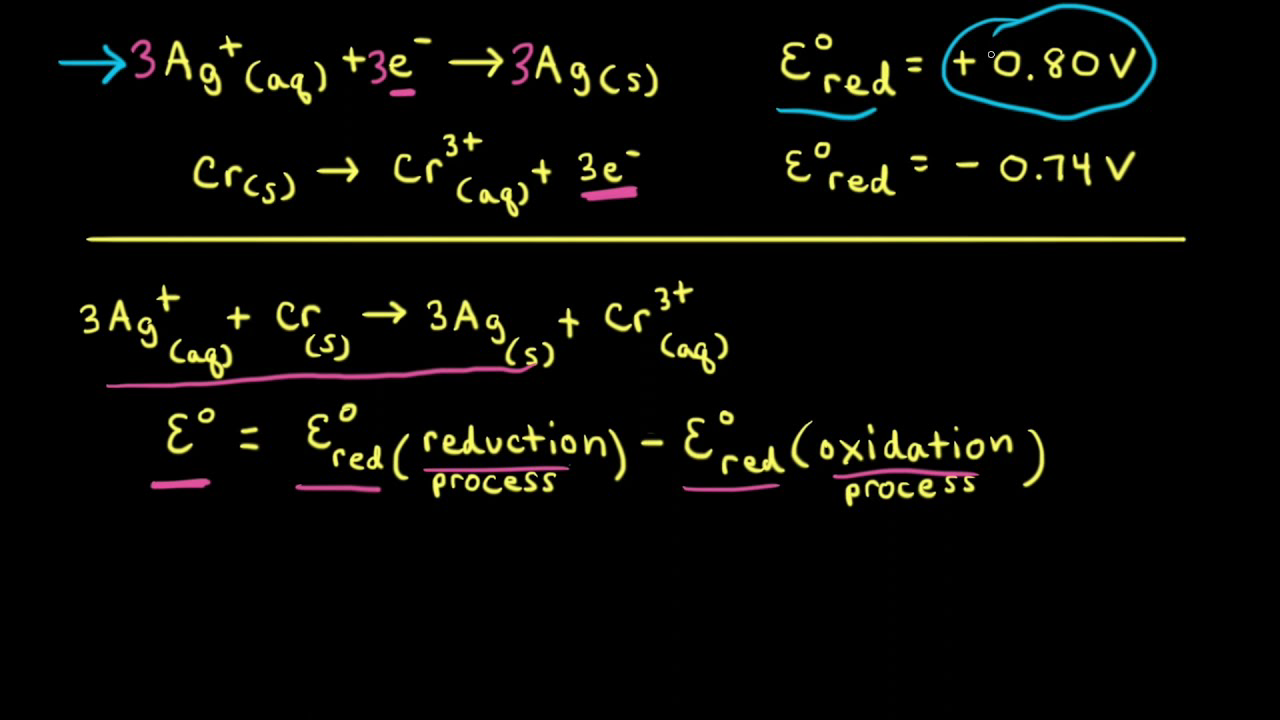





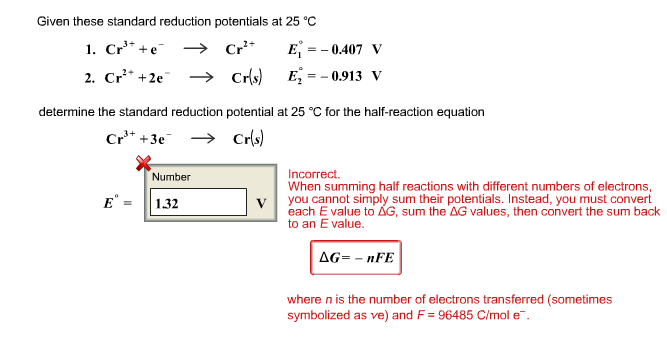
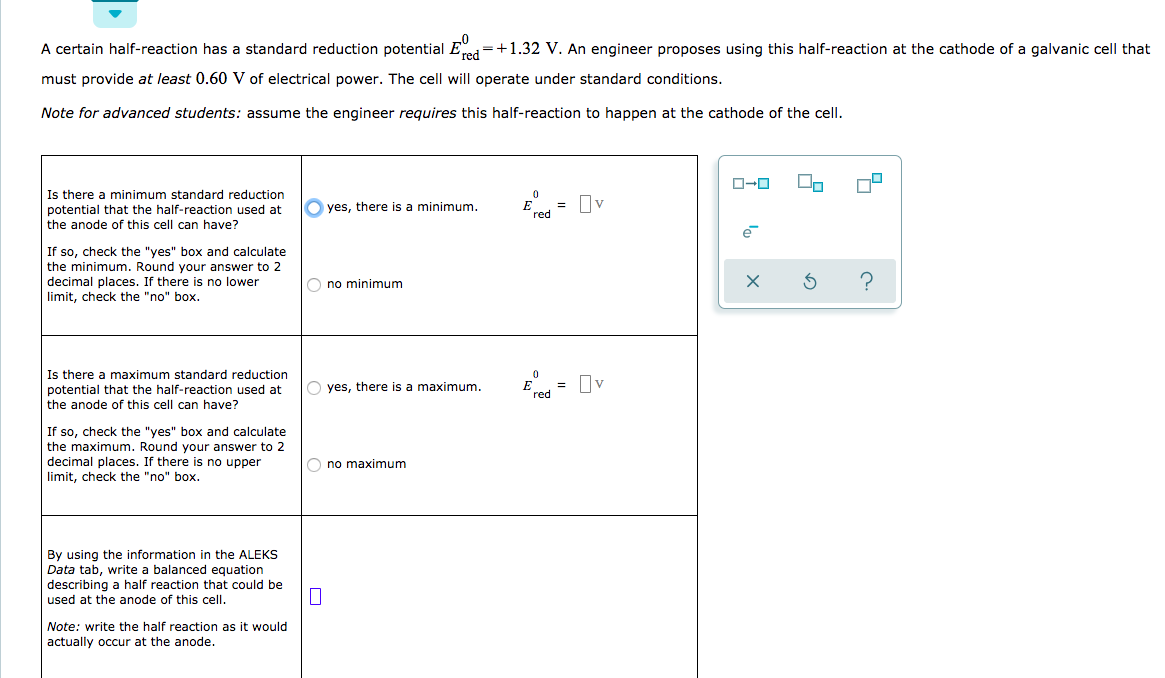
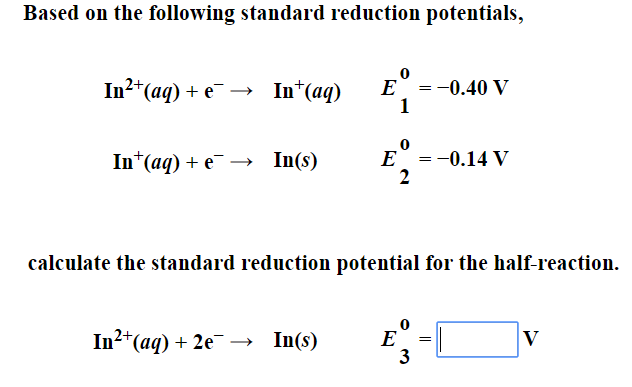


![ANSWERED] A certain half-reaction has a standard re... - Physical Chemistry ANSWERED] A certain half-reaction has a standard re... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/59671895-1659273579.31138.jpeg)