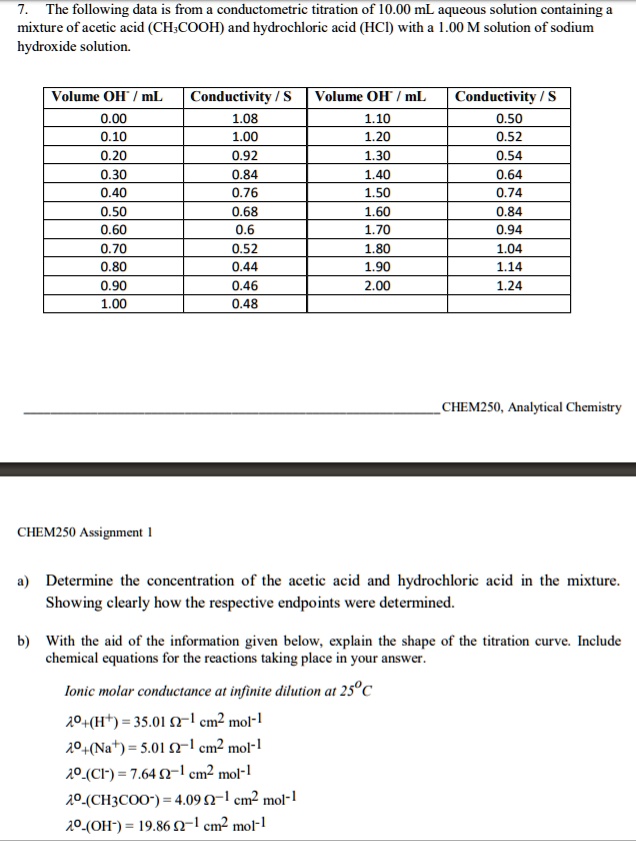
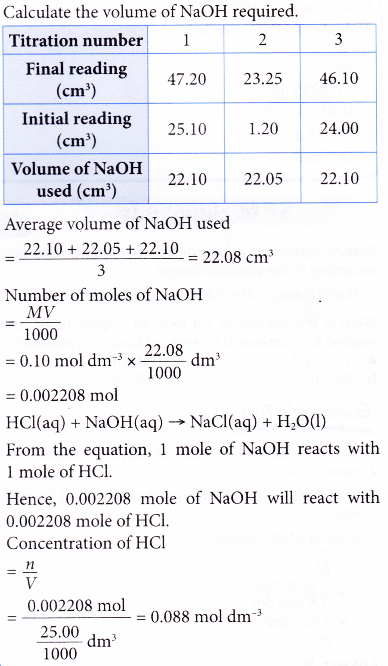
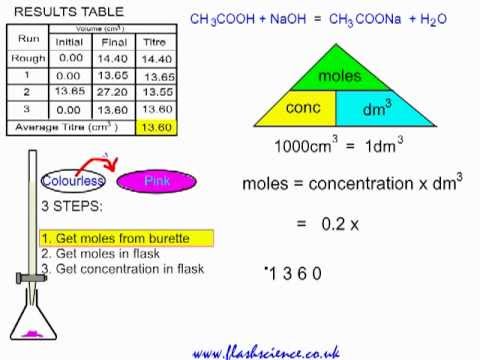
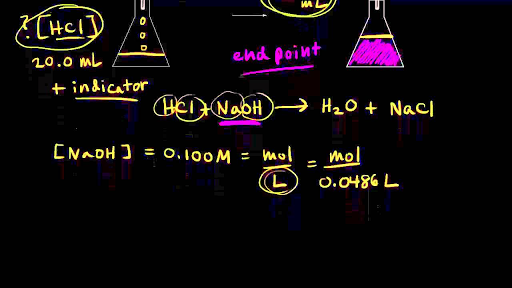
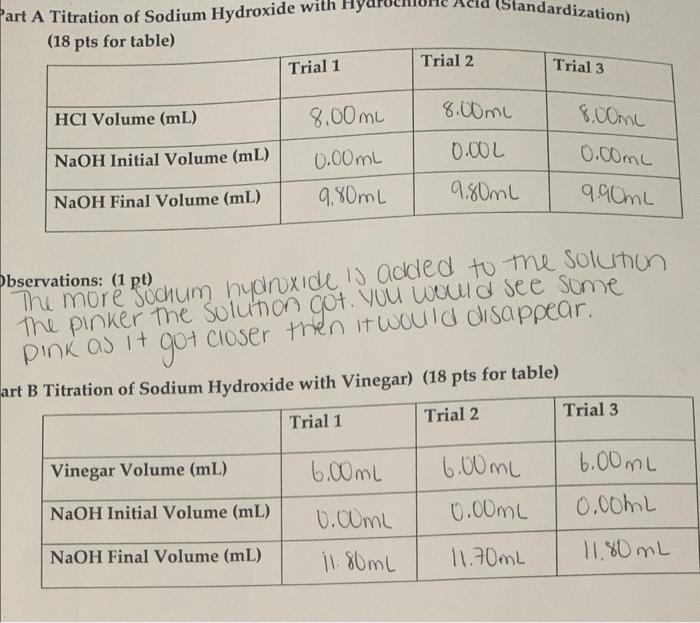
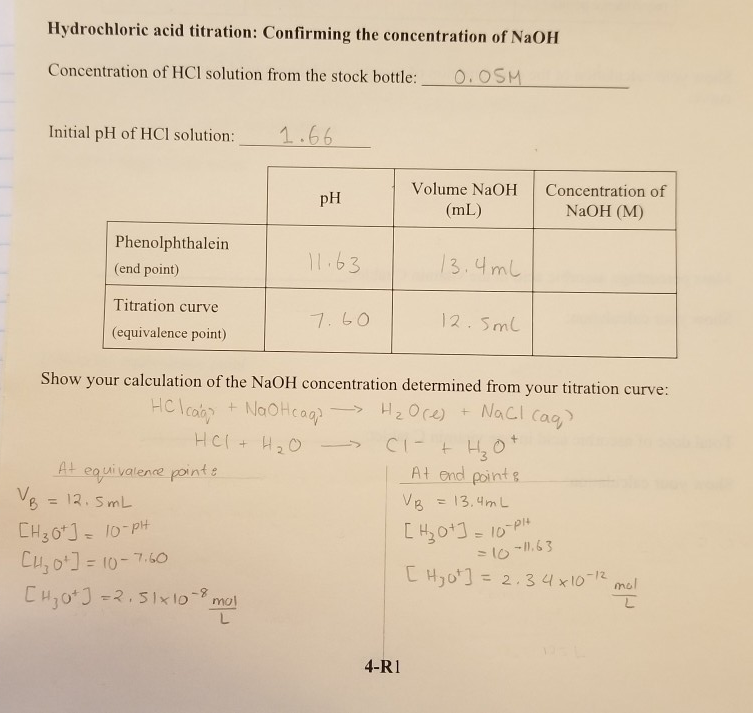
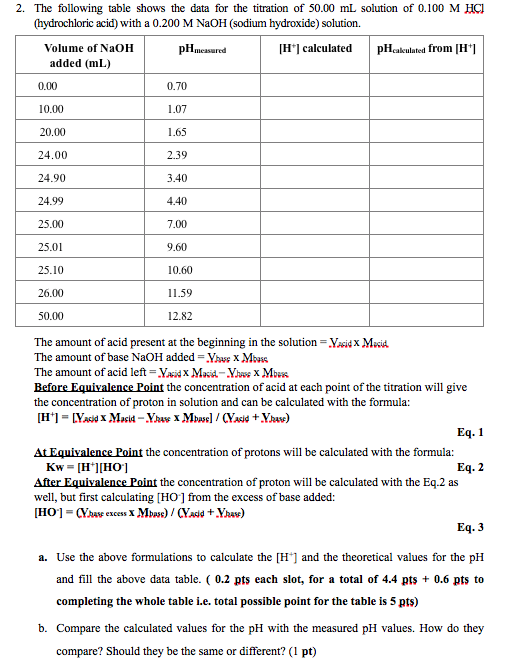
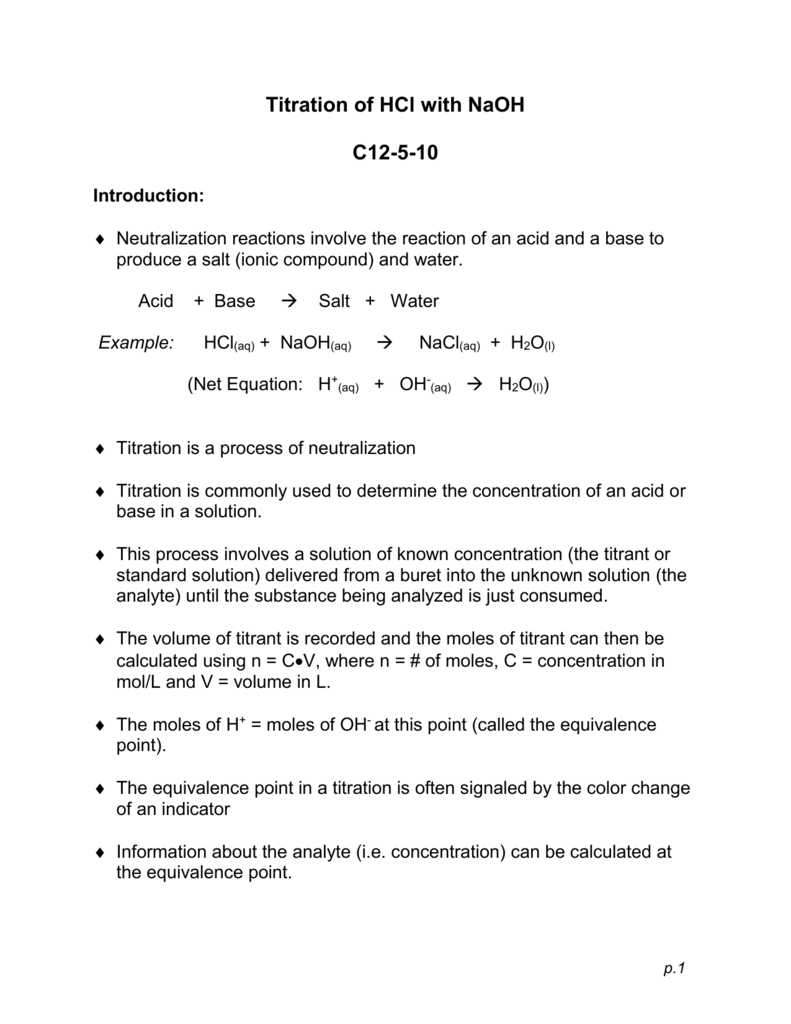
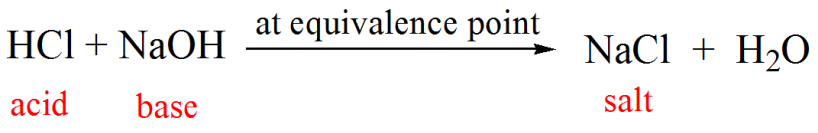Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.

SOLVED: Molarities of Solution:: Solution Molarity NAOH LlolsM HCI MldleH HC,HO: L10XM Titration of Hydrochloric Acid: mL base needed to reach the equivalence point: 44Im Show how you detenined this volume from

SOLVED: 0.200 M sodium hydroxide (NaOH) being added to 30 mL of hydrochloric acid (HCl) of unknown concentration. Your goal is to measure the volume of sodium hydroxide needed to neutralize the